Molecular and Reproductive Toxicology
The Research Group for Molecular and Reproductive Toxicology studies how environmental chemicals can disrupt normal development and cause disease. The group has a particular focus on endocrine disruptors and how exposure to these can cause reproductive or cognitive disorders, alongside developing new approach methodologies for chemical testing. The ultimate goal is to safeguard human health by improving chemical safety testing and risk assessment.
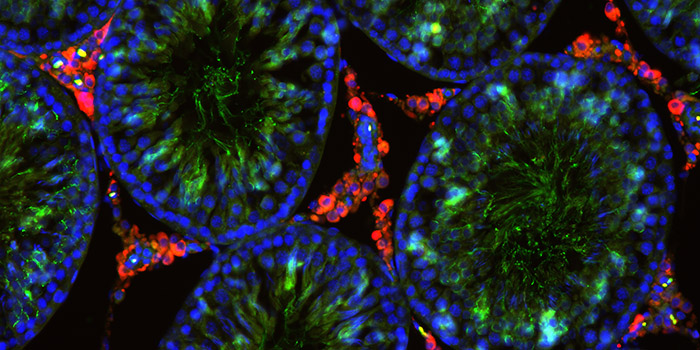
The aim of the group’s work is to elucidate how chemicals contribute to human disease, as well as how chemical regulation can account for complex human exposure scenarios.
Approaches and methods
- Endocrine disruption and development: investigate how chemical perturbations of hormone signaling lead to adverse reproductive and neurodevelopmental outcomes in humans.
- Reproductive toxicology: identify vulnerable windows during development and the biological processes that can affect reproductive health using animal models.
- Mechanistic toxicology and AOPs: develop toxicity-relevant Adverse Outcome Pathways and apply them for mechanism-based risk assessment.
- New Approach Methodologies (NAMs): develop human-relevant methods using cell-lines, primary cells, and ex vivo cultures for chemical screening to prioritize high-tier testing and to provide mechanistic knowledge of toxicological effects.
- Molecular toxicology: use state-of-the-art molecular biology methods, -omics technologies, and image analysis to decipher mechanisms of action.
- Regulatory science and test guidelines: provide scientific expertise for hazard identification and test guideline development, and contribute to expert panels, ensuring that methods keep pace with new regulatory requirements.
- Interdisciplinary integration: use biological analyses, cell-based laboratory assays, and chemical risk assessment to bridge basic research and regulatory practice, in Denmark and internationally.
Collaborations and impact
Contact
Terje Svingen Professor, Head of Research Group Mobile: +45 93518880 tesv@food.dtu.dk