Genomic EQA
Genomic Proficiency Testing for African laboratories
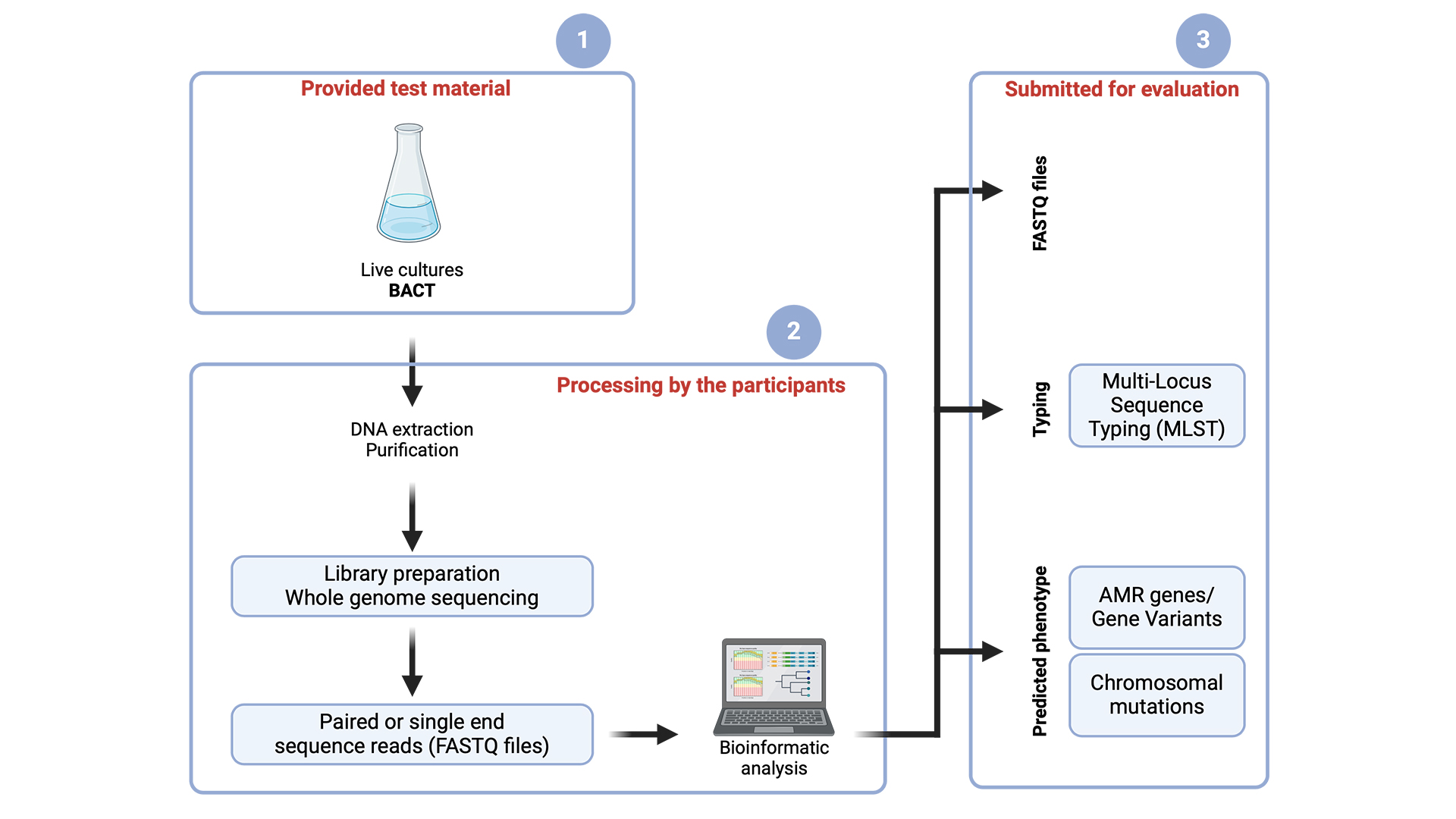
Why participate in the DTU Genomic Proficiency test?
The proficiency test (PT) allows participating laboratories the opportunity to evaluate the reliability and quality of their laboratory's results in DNA preparation, sequencing, and analysis.What is the DTU Genomic Proficiency Test?
This inter-laboratory performance test is provided to facilitate harmonisation and standardisation in whole genome sequencing and data analysis, with the aim of producing comparable data for monitoring and research purposes.
The PT consists of two parts to include assessing (1a) the laboratory's DNA preparation and sequencing procedures and (1b) the laboratory's sequencing output.
The proficiency tests focus on several bacterial species that might vary from year to year. Participants have the option to sign up for all species relevant to their laboratory.
Cost for participation
For laboratories associated with Fleming Fund Country Grants, members of the Pasteur Institute’s SARA network, and laboratories that are members of the African CDC Pathogen Genomics Initiative network there is no participation fee for the Genomic Proficiency test. SeqAfrica will cover the cost of participation and shipping of bacterial isolates to the participating lab. Participating laboratories are, however, expected to cover all expenses related to the handling and sequencing of the test strains provided in the proficiency test.
Protocol and further information
The protocol including appendices will be made available for download via this website: About the Genomic Proficiency Test 2024
Additional information relevant for participants will be sent directly by email.
If your institution requires a permit in order to receive a UN3373 shipment from Denmark, please apply for an import permit to receive the following bacterial cultures (according to your level of participation): UN3373, Biological Substance, Category B: Two E. coli strains, two S. aureus strains and two Enterococcus strains (note: some of these strains may be carbapenemase-producing). In order to minimize delays, we ask you to send a valid import permit to the PT coordinator (see below) as soon as possible.
Each participating laboratory will receive an individual summary of its performance. An overall summary of the results will be published. Individual results will be anonymized, and only the PT-organizers will have access to your laboratory’s results. Due to the anonymity of results, authors and co-authors of publication(s) will be those who have contributed to the preparation and execution of the proficiency test.
How to participate?
Sign-up for the DTU Genomic PT 2024 here: https://ec.europa.eu/eusurvey/runner/9eee5f58-f14f-439b-1019-0250ec705800Timeline
Deadline for sign-up: 23 August 2024.
Test material (bacterial isolates) will be shipped from National Food Institute DTU mid-October 2024.
The PT material will be sent as ‘UN3373 Biological Substance Category B’ (without temperature control).
Deadline for result submission will be 9 December 2024 at 4 pm.
Previous DTU Genomic Proficiency Tests
More information and protocols from previous DTU genomic PTs are available through the links below:
DTU Genomic PT 2023
DTU Genomic PT 2022